-
Rani Therapeutics Reports Third Quarter 2023 Financial Results; Provides Corporate Update
المصدر: Nasdaq GlobeNewswire / 08 نوفمبر 2023 16:05:02 America/New_York
- Cash runway extended into 2025 -
- Announced strategic program prioritization, expansion of manufacturing and plans to streamline business operations -
- Initiated Phase 1 clinical trial of RT-111, a RaniPill GO containing ustekinumab biosimilar CT-P43, with topline results expected in 1Q 2024 -
- Initiation of Phase 2 clinical trial of RT-102, a RaniPill GO containing teriparatide for osteoporosis, expected by the year-end 2023 -
- Announced RaniPill capsule was well-tolerated in 60-day, repeat oral-administration GLP safety study -
- Announced successful drug delivery with RaniPill HC high-capacity capsule in multiple preclinical studies with cumulative >90% success rate -
SAN JOSE, Calif., Nov. 08, 2023 (GLOBE NEWSWIRE) -- Rani Therapeutics Holdings, Inc. (“Rani Therapeutics” or “Rani”) (Nasdaq: RANI), a clinical-stage biotherapeutics company focused on the oral delivery of biologics and drugs, today reported financial results for the third quarter ended September 30, 2023, and provided a corporate update.
“In the third quarter, we made important decisions to drive forward our key programs to create long-term value for our shareholders and extend our cash runway into 2025. We announced a strategic prioritization of our RT-102 and RT-111 programs, as well as development of the RaniPill HC to be Phase 1 ready,” said Talat Imran, Chief Executive Officer of Rani Therapeutics. “We are highly encouraged by the continued development of the RaniPill HC, a novel high-capacity capsule capable of delivering up to a 500%-plus higher drug payload than Rani’s existing oral biologics capsule. We have completed multiple preclinical studies of the RaniPill HC with antibodies and other molecules. Our goal is to get this capsule ready for Phase 1 studies in the clinic and we look forward to potential partnering opportunities involving the RaniPill HC.”
Third Quarter or Subsequent Highlights:
- Announced Strategic Program Prioritization, Expansion of Manufacturing and Plans to Streamline Business operations to Support Near-Term Value Drivers and Long-Term Growth of the RaniPill Technology Platform. The plans include strategic prioritization of its key development programs, RT-102, RT-111 and the RaniPill HC and expansion of its manufacturing footprint to support increased scale and partnerships, and cost reduction initiatives that align with Rani’s near-term goals. Development of RT-101 will be discontinued, while the development of RT-105 and RT-110 programs will be paused. In addition, Rani will reduce its workforce by approximately 25%. Anticipated cost savings are expected to support Rani’s operating plans into 2025.
- Completed 60-Day, Repeat Oral-Administration GLP Safety Study. In October 2023, Rani announced preclinical data from a 60-day, repeat oral-administration GLP safety study of the RaniPill capsule in healthy animals. The RaniPill capsule was well-tolerated with no treatment-related adverse events and all animals remained clinically healthy throughout the study.
- Presented an Abstract on RT-102 at the 2023 Annual Meeting of the American Society for Bone and Mineral Research. The abstract focused on the safe and reliable delivery of teriparatide with high bioavailability through daily administration of an oral robotic pill (RT-102) in female volunteers.
- Initiated Phase 1 Clinical Trial of RT-111 (RaniPill Containing Ustekinumab Biosimilar, CT-P43). In September 2023, Rani announced the initiation of a Phase 1 clinical trial to evaluate the safety and tolerability of RT-111, an orally administered RaniPill GO capsule containing an ustekinumab biosimilar, CT-P43. Currently, ustekinumab is available only as a subcutaneous injection. In preclinical testing of RT-111 in animal models, the RaniPill delivered ustekinumab biosimilar orally with bioavailability comparable to subcutaneous injection. Topline results from this study are expected early in the first quarter of 2024.
- Announced Successful Drug Delivery of High-Capacity Pill in Preclinical Studies. In September and October 2023, Rani announced results from multiple preclinical studies of the RaniPill HC, a version of the RaniPill capsule capable of delivering up to a 500%-plus higher drug payload than Rani’s existing oral biologics capsule. In such studies, the RaniPill HC achieved successful drug delivery with a cumulative >90% success rate.
Development Update
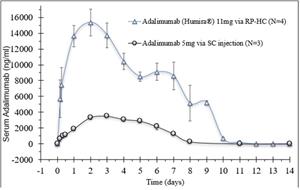
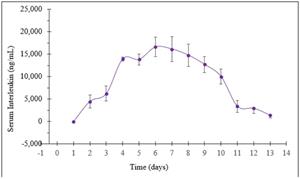
- Rani has completed two preclinical studies of the RaniPill HC with antibodies, adalimumab and an undisclosed interleukin (“Interleukin”). In the two antibody studies, the RaniPill HC achieved an oral delivery success rate of 100% (10/10). In one study, Rani tracked the serum concentrations of adalimumab, following the oral administration of the enteric-coated, RaniPill HC capsule containing 11mg of Humira (adalimumab) to four canine models. In the second study, Rani tracked the serum concentrations of the Interleukin, following the oral administration of the enteric-coated RaniPill HC capsule containing 16.5mg of Interleukin to six canine models. In both studies, the RaniPill HC was well-tolerated, all animals remained healthy throughout the study period with no clinical findings or adverse events, and all device remnants were excreted normally without sequelae.
- Comparing the pharmacokinetic results of 11mg of adalimumab delivered via the RaniPill HC (N=4) with historical pharmacokinetic data generated by Rani with 5mg of an adalimumab biosimilar (GP2017) delivered via subcutaneous injection (N=3), there is a higher estimated bioavailability of adalimumab delivered via the RaniPill HC relative to the subcutaneous injection route.
Adalimumab 11mg via RaniPill HC vs Adalimumab Biosimilar 5mg via Subcutaneous Injection
All Data are Means ± SE
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/17476063-074d-43a1-9815-963636ade293
Pharmacokinetics of Interleukin (16.5mg) Delivered Orally via RaniPill HC Capsules to Awake Canines (N=6)
All Data are Means ± SE
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d742871b-dcba-4f9b-8afa-f7bf8e66783d
Preliminary preclinical testing supports the potential for the RaniPill HC to have high reliability and initial analysis of drug delivery via the RaniPill HC shows a potential for mimicking parenteral (subcutaneous) administration. Rani expects to continue preclinical testing of RaniPill HC to confirm the preliminary reliability rate and optimize device performance with a goal for the RaniPill HC to be ready to advance into the clinic in the second half of 2024.
Near-Term Milestone Expectations:
- Initiation of Phase 2 clinical trial of RT-102, a RaniPill GO containing teriparatide for osteoporosis, expected by year-end 2023.
- Topline results of Phase 1 clinical trial of RT-111, a RaniPill GO containing ustekinumab biosimilar CT-P43, expected in the first quarter of 2024.
- Development of RaniPill HC to be ready for potential Phase 1 clinical trials in the second half of 2024.
Third Quarter Financial Results:
- Cash, cash equivalents and marketable securities as of September 30, 2023, totaled $60.5 million, compared to cash, cash equivalents and marketable securities of $98.5 million as of December 31, 2022. Rani expects its cash, cash equivalents and marketable securities to be sufficient to fund its operations through at least the next twelve months.
- Research and development expenses were $11.2 million for the three months ended September 30, 2023, compared to $9.1 million for the three months ended September 30, 2022. The increase was primarily attributed to higher third-party services expense of $2.1 million due to pre-clinical and clinical development activities.
- General and administrative expenses were $6.6 million for the three months ended September 30, 2023, compared to $7.2 million for the three months ended September 30, 2022. The decrease was primarily attributed to third-party services of $0.7 million related to support for compliance with public company requirements.
- Net loss for the three months ended September 30, 2023 was $18.3 million, compared to $16.2 million for the comparable period in 2022, including stock-based compensation expense of $5.0 million for the three months ended September 30, 2023 compared to $4.4 million for the comparable period in 2022.
About Rani Therapeutics
Rani Therapeutics is a clinical-stage biotherapeutics company focused on advancing technologies to enable the development of orally administered biologics and drugs. Rani has developed the RaniPill capsule, which is a novel, proprietary and patented platform technology, intended to replace subcutaneous injection or intravenous infusion of biologics and drugs with oral dosing. Rani is progressing two RaniPill capsules, the RaniPill GO and the RaniPill HC. Rani has successfully conducted several preclinical and clinical studies to evaluate safety, tolerability and bioavailability using RaniPill capsule technology. For more information, visit ranitherapeutics.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, the advancement of Rani’s pipeline and RaniPill platform technology including the RaniPill HC, the potential of the RaniPill HC to deliver 500%-plus higher drug payload than Rani’s existing oral biologics capsule, the expected initiation of a Phase 2 clinical trial of RT-102 in 2023, the ability to confirm preliminary reliability and optimize performance of the RaniPill HC, the expected timing of topline results from the RT-111 Phase 1 clinical trial in the first quarter of 2024, the expected readiness of the RaniPill HC for clinical development in the second half of 2024, the ability of expanded manufacturing footpoint to support scaling of manufacturing and partnering, the ability to streamline its business operations and to realize the cost-savings contemplated by such streamlining of business operations, reduction in workforce and other initiatives announced by Rani, the potential for the RaniPill HC to have high reliability and mimic parenteral (subcutaneous) administration, the potential for partner opportunities with the RaniPill HC, customer acceptance of the RaniPill capsule technology, the potential benefits of the RaniPill capsule technology, cash sufficiency forecast, and Rani’s growth as a company. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “capable of,” “potential,” “expects,” “with a goal for,” “anticipate” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Rani’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with Rani’s business in general and the other risks described in Rani’s filings with the Securities and Exchange Commission, including Rani’s annual report on Form 10-K for the year ended December 31, 2022, and subsequent filings and reports by Rani. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Rani undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Trademarks
Trade names, trademarks and service marks of other companies appearing in this press release are the property of their respective owners. Solely for convenience, the trademarks and trade names referred to in this press release appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames.
Investor Contact:
investors@ranitherapeutics.comMedia Contact:
media@ranitherapeutics.comRANI THERAPEUTICS HOLDINGS, INC
Condensed Consolidated Balance Sheets
(In thousands, except par value)September 30, December 31, 2023 2022 (Unaudited) Assets Current assets: Cash and cash equivalents $ 4,972 $ 27,007 Marketable securities 55,554 71,475 Prepaid expenses and other current assets 2,696 2,442 Total current assets 63,222 100,924 Property and equipment, net 6,255 6,038 Operating lease right-of-use asset 959 1,065 Total assets $ 70,436 $ 108,027 Liabilities and Stockholders' Equity Current liabilities: Accounts payable $ 1,405 $ 1,460 Accrued expenses and other current liabilities 3,930 2,349 Current portion of long-term debt 1,222 — Current portion of operating lease liability 788 1,006 Total current liabilities 7,345 4,815 Long-term debt, less current portion 28,101 29,149 Operating lease liability, less current portion 171 59 Total liabilities 35,617 34,023 Stockholders' equity: Preferred stock, $0.0001 par value - 20,000 shares authorized; none issued and outstanding as of September 30, 2023 and December 31, 2022 — — Class A common stock, $0.0001 par value - 800,000 shares authorized; 25,876 and 25,295 issued and outstanding as of September 30, 2023 and December 31, 2022, respectively 3 3 Class B common stock, $0.0001 par value - 40,000 shares authorized; 24,116 issued and outstanding as of September 30, 2023 and December 31, 2022 2 2 Class C common stock, $0.0001 par value - 20,000 shares authorized; none issued and outstanding as of September 30, 2023 and December 31, 2022 — — Additional paid-in capital 83,380 75,842 Accumulated other comprehensive loss (41 ) (73 ) Accumulated deficit (65,791 ) (38,919 ) Total stockholders' equity attributable to Rani Therapeutics Holdings, Inc. 17,553 36,855 Non-controlling interest 17,266 37,149 Total stockholders' equity 34,819 74,004 Total liabilities and stockholders' equity $ 70,436 $ 108,027 RANI THERAPEUTICS HOLDINGS, INC
Condensed Consolidated Statements of Operations
(In thousands, except per share amounts)
(Unaudited)Three Months Ended September 30, Nine Months Ended September 30, 2023 2022 2023 2022 Operating expenses Research and development $ 11,220 $ 9,103 $ 32,018 $ 26,221 General and administrative 6,635 7,239 20,647 19,748 Total operating expenses $ 17,855 $ 16,342 $ 52,665 $ 45,969 Loss from operations (17,855 ) (16,342 ) (52,665 ) (45,969 ) Other income (expense), net Interest income and other, net 839 379 2,626 430 Interest expense and other, net (1,316 ) (352 ) (3,789 ) (352 ) Loss before income taxes (18,332 ) (16,315 ) (53,828 ) (45,891 ) Income tax expense — 107 — (111 ) Net loss $ (18,332 ) $ (16,208 ) $ (53,828 ) $ (46,002 ) Net loss attributable to non-controlling interest (9,135 ) (8,253 ) (26,956 ) (24,200 ) Net loss attributable to Rani Therapeutics Holdings, Inc. $ (9,197 ) $ (7,955 ) $ (26,872 ) $ (21,802 ) Net loss per Class A common share attributable to Rani Therapeutics Holdings, Inc., basic and diluted $ (0.36 ) $ (0.33 ) $ (1.06 ) $ (0.93 ) Weighted-average Class A common shares outstanding—basic and diluted 25,552 24,468 25,380 23,449 
Adalimumab 11mg via RaniPill HC vs Adalimumab Biosimilar 5mg via Subcutaneous Injection
Adalimumab 11mg via RaniPill HC vs Adalimumab Biosimilar 5mg via Subcutaneous Injection
Pharmacokinetics of Interleukin (16.5mg) Delivered Orally via RaniPill HC Capsules to Awake Canines (N=6)
Pharmacokinetics of Interleukin (16.5mg) Delivered Orally via RaniPill HC Capsules to Awake Canines (N=6)